- Instruction sheets
- Hits: 5575
Immunoassay Hormonal analysis Instruction sheet
|
Immunoassay Hormonal analysis Instruction sheet |
|||||||
|
INS-INP-003- Immunoassay Hormonal analysis Instruction sheet-V2.0 |
Version#: 2.0 |
Issue date: 2025 |
Language : English-Arabic |
|
|||
|
Subject form: Immunoassay Hormonal analysis Instruction sheet |
Subject file: INS-INP-002-Immunoassay Hormonal analysis Instruction sheet-V2.0 |
Pages # :4 |
Related SOPs&Documents: |
||||
|
Prepared by: Ziyad Alkhdour |
Verified by: Duaa Mushalah |
Qualified by: Sawsan Khawaja |
Approved by: Ziyad Alkhdour |
||||
Dear Participant:
Enclosed is your Clinical Immunoassay Hormonal specimen number (xxx) tested negative for hepatitis and HIV.
Specimen Type
Lyophilized Human Serum
Precautions BIOHAZARD: Human source material. Handle as if potentially infectious
Specimen Storage
Keep the specimen lyophilized until use and store it at 2-8 °C.
Intended use:
EQAS Clinical Immunoassay Hormonal Control sample is intended for use as a quality control Clinical Immunoassay Hormonal to monitor the accuracy of hormone test procedures for the analytes present in tested sample.
Reconstituting Lyophilized EQAS Material
The process of reconstitution involves adding a specified volume of distilled water to lyophilized QC material. The water should completely dissolve the lyophilized contents, giving a liquid solution, which is ready for analysis.
Reconstitution is a straightforward process, but requires a high level of precision. Small errors can have serious implications to the reconstituted material:
- If too much water is pipetted during reconstitution, the material will be heavily diluted and results will be lower than expected
- If too little water is pipetted during reconstitution, the material will not be sufficiently diluted, and results will be higher than expected
- If the correct volume of water is pipetted, but a small amount of water gets stuck in the pipette tip due to poor pipetting technique, results will be higher than expected
If a lyophilized control has been reconstituted incorrectly the contents of the vial will be wasted. It is therefore vitally important that controls are reconstituted with care.
Materials and Methods Required
The list of requirements for an accurate and consistent reconstitution technique is not extensive, but each requirement is vital. Labs should have:
- Calibrated volumetric pipettes
- Sterile, appropriately sized pipette tips
- Distilled water
- Technician with good pipetting technique
- Lyophilized EQAS stored at refrigerator (2-8 °C)
How to Reconstitute Lyophilised EQAS Material
Each different lyophilised control may require slightly different preparation, always refer to the instructions for use before reconstituting control material.
- Place the vial of lyophilized EQAS on a flat surface, carefully remove the lid and the rubber stopper making sure not to spill any material
- Using a calibrated pipette and sterilized pipette tip, add exactly 1ml of distilled water directly into the EQAS vial, ensuring no water is left in the pipette tip, or on the rim/side of the vial
- Place the rubber stopper and lid firmly back onto the EQAS vial, and leave to stand for 30 minutes
- After 30 minutes, gently invert the EQAS vial 10-15 times to ensure the contents is completely dissolved, making sure to avoid the formation of foam. It is important that you DO NOT SHAKE the vial. Alternatively place the vial on a roller for 30 minutes to ensure the contents is thoroughly mixed
- Once satisfied all material has been completely dissolved, proceed to use the EQAS product in accordance with the ‘sample’ section of the individual analyzer application
- Treat this specimen exactly as you do with any other clinical specimen.
- Complete the assay for analytes indicated on the accompanying Results Return Sheet.
- Use the methods and equipment reported in the methodology Registration sheet.
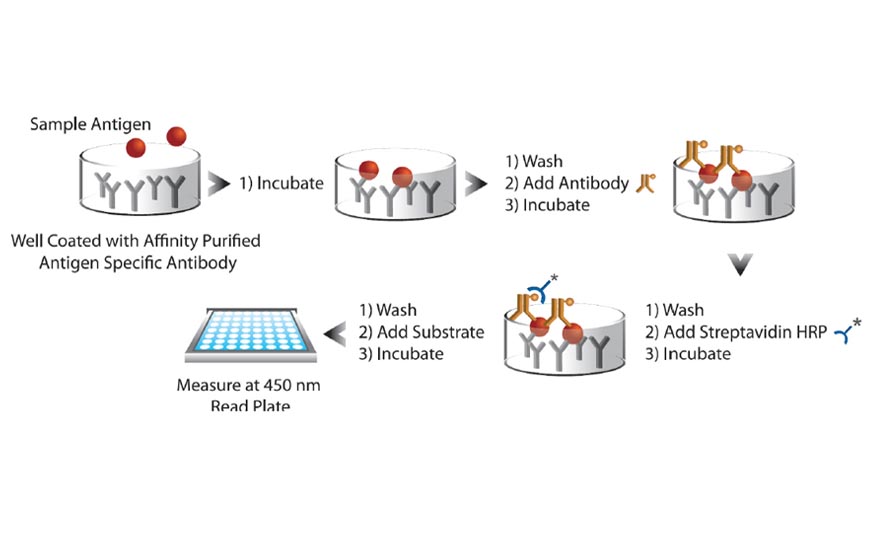
- Perform the following test items (TSH, FSH, LH, Free T3, Free T4, Prolactin, Vit-D, B-12,Ferritin and β-hCG,) by your routine methods during your normal workload.
- On the Results Return Sheet record the date the sample was received and the date the sample was analyzed.
- Write your results clearly in the appropriate result space ensuring they are in the units indicated.
- Once finished, freeze any unused material in a small aliquot for further use. It is good practice to label the vial with the date of reconstitution to prevent the use of material outside of the recommended stability period
- Prior to reusing lyophilised material, mix the contents thoroughly by gentle inversion, as highlighted in Step 4
Stability
Typical stability of constituents in reconstituted serum: at +25°C at least 8 hours; refrigerated at least 3 days; at -20°C at least 1 month (to be frozen only once).
Return your results to EQAS office:
* Through the web site of EQAS (www.eqas.alquds.edu)
* By fax: 02/2411149
* By E-mail: This email address is being protected from spambots. You need JavaScript enabled to view it.
* By telephone: 02/2414076 or 0598583807
* By WhatsApp 970598583807
Method, reagent and instrument are entered into the fields. You will find the analysis key for methods and the key for reagents in this commentary. The entries in the indicated fields are important for the correct classification of results for a defined group for assessment. If other methods, reagents, or instruments have been used, which are not listed in the key, please indicate the reagents used. The fields for results are for entering your analytical results
For consultation and/or questions please contact
Mr. Ziyad Al khdour On Sunday. thru Thursday. at 9:00- 15:00
Additional Considerations
It is important to remember that there may be slightly different reconstitution requirements for different EQAS material. For this reason, it is vital that the instructions provided on the Website are closely followed.
If you have any further questions regarding lyophilised controls or would like to contact us, please do so by emailing us at This email address is being protected from spambots. You need JavaScript enabled to view it. or you can ask specifically
Mr. Ziyad Al khdour On Sunday- Thursday. at 9:00- 15:00
Written by Ziyad Khdour



